How Samsung’s Engineering Feat Became a Catalyst for Scientific and Industry Advancement [Interview on Real Quantum Dots Part 2.]
on April 4, 2025
“Samsung’s QLED technology played a crucial role in bringing quantum dots to the level of recognition needed for the Nobel Prize in Chemistry.”
— Taeghwan Hyeon, Seoul National University
Quantum dots have been at the forefront of display innovation over the past decade, delivering some of the most accurate color reproduction among existing materials. In 2015, Samsung Electronics paved the way for the commercialization of quantum dots with the launch of SUHD TVs — a breakthrough that moved beyond the use of cadmium (Cd), a heavy metal traditionally utilized in quantum dot synthesis, by introducing the world’s first no-cadmium quantum dot technology.
The academic world took notice. The successful commercialization of cadmium-free quantum dot TVs not only set a new direction for research and development but also played a pivotal role in the awarding of the 2023 Nobel Prize in Chemistry for the discovery and synthesis of quantum dots.
Following Part 1, Samsung Newsroom uncovers how Samsung has contributed to academia through groundbreaking advances in material innovation.

▲ (From left) Taeghwan Hyeon, Doh Chang Lee and Sanghyun Sohn
Why Cadmium Was the Starting Point for Quantum Dot Research
“I was truly impressed that Samsung succeeded in commercializing a no-cadmium quantum dot display product.”
— Taeghwan Hyeon, Seoul National University
Quantum dots began attracting scientific interest in the 1980s when Aleksey Yekimov, former Chief Scientist at Nanocrystals Technology Inc., and Louis E. Brus, a professor emeritus in the Department of Chemistry at Columbia University, each published their researches on the quantum confinement effect and the size-dependent optical properties of quantum dots.
Momentum accelerated in 1993 when Moungi Bawendi, a professor in the Department of Chemistry at the Massachusetts Institute of Technology (MIT), developed a reliable method for synthesizing quantum dots. In 2001, Taeghwan Hyeon, a distinguished professor in the Department of Chemical and Biological Engineering at Seoul National University (SNU), invented the “heat-up process” — a technique for producing uniform nanoparticles without the need for size-selective separation. In 2004, Hyeon published a scalable production method in the academic journal Nature Materials — a discovery widely regarded as a potential game changer in the industry.

▲ Taeghwan Hyeon
However, these efforts did not immediately lead to commercialization. At the time, quantum dots relied heavily on cadmium(Cd) as a core material — a substance known to be harmful to humans and designated as a restricted material under the European Union’s Restriction of Hazardous Substances (RoHS) Directive.
“Currently, the only materials capable of reliably producing quantum dots are cadmium selenide (CdSe) and indium phosphide (InP),” explained Hyeon. “Cadmium selenide, the conventional quantum dot material, is a compound of group II and group VI elements, while indium phosphide is formed from group III and group V elements. Synthesizing quantum dots from group II and VI elements is relatively straightforward, but combining group III and V elements is chemically much more complex.”
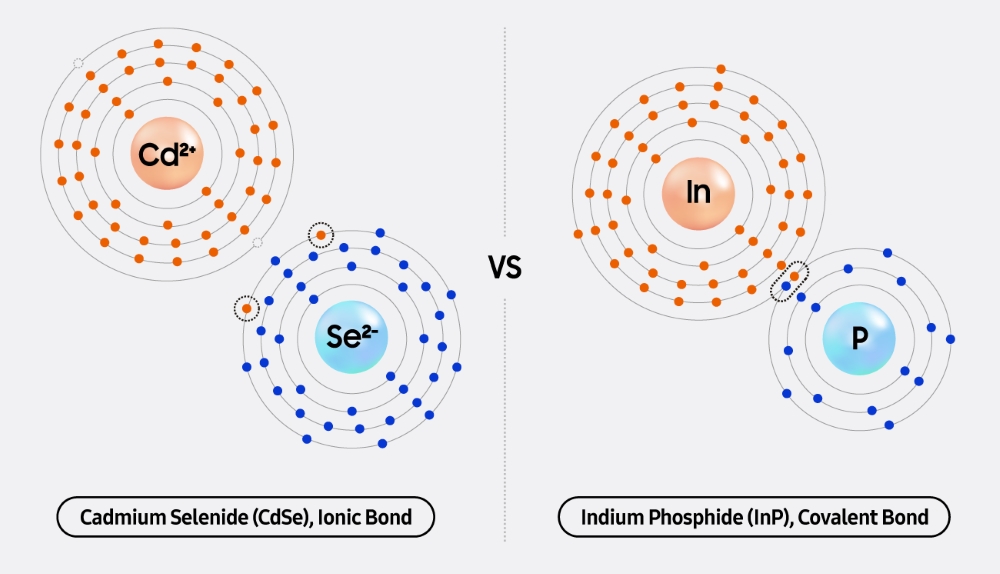
▲ A comparison of cadmium-based quantum dots with ionic bonds and indium-based quantum dots with covalent bonds
Cadmium, an element with two valence electrons, forms strong ionic bonds1 with elements like selenium (Se), sulfur (S) and tellurium (Te) — each of which has six valence electrons. These combinations result in stable semiconductors, known as II–VI semiconductors, materials that have long been favored in research for their ability to produce high-quality nanocrystals even at relatively low temperatures. As a result, the use of cadmium in quantum dot synthesis was considered an academic standard for many years.
In contrast, indium (In) — an alternative to cadmium with three valence electrons — forms covalent bonds2 with elements such as phosphorus (P), which has five valence electrons. Covalent bonds are generally less stable than ionic bonds and have a directional nature, increasing the likelihood of defects during nanocrystal synthesis. These characteristics have made indium a challenging material to work with in both research and mass production.
“It is difficult to achieve high crystallinity in quantum dots made from indium phosphide,” Lee noted. “A complex and demanding synthesis process is required to meet the quality standards necessary for commercialization.”
No Compromise – From Breakthrough to Mass Production
“There is simply no room for compromise when it comes to consumer safety.”
— Sanghyun Sohn, Samsung Electronics
Samsung, however, took a different approach.
“We had been researching and developing quantum dot technology since 2001,” said Sanghyun Sohn, Head of Advanced Display Lab, Visual Display (VD) Business at Samsung Electronics. “But early on, we determined that cadmium — which is harmful to the human body — was not suitable for commercialization. While regulations in some countries technically allow up to 100 parts per million (ppm) of cadmium in electronic products, Samsung adopted a zero-cadmium policy from the start. No cadmium, no compromise — that was our strategy. There is simply no room for compromise when it comes to consumer safety.”

▲ Sanghyun Sohn
Samsung’s long-standing commitment to its principle of “No Compromise on Safety” came to the forefront in 2014 when the company successfully developed the world’s first no-cadmium quantum dot material. To ensure both durability and image quality, Samsung introduced a triple-layer protective coating technology that shields indium phosphide nanoparticles from external factors such as oxygen and light. The following year, Samsung launched the world’s first commercial SUHD TV with no-cadmium quantum dots — a paradigm shift in the display industry and the culmination of research efforts that began in the early 2000s.
“Indium phosphide-based quantum dots are inherently unstable and more difficult to synthesize compared to their cadmium-based counterparts, initially achieving only about 80% of the performance of cadmium-based quantum dots,” said Sohn. “However, through an intensive development process at the Samsung Advanced Institute of Technology (SAIT), we successfully raised performance to 100% and ensured reliability for more than 10 years.”

▲ The three components of quantum dots
Quantum dots found in Samsung QLEDs are composed of three key components — a core, where light is emitted; a shell, which protects the core and stabilizes its structure; and a ligand, a polymer coating that enhances oxidation stability outside the shell. The essence of quantum dot technology lies in the seamless integration of these three elements, an advanced industrial process that spans from material acquisition and synthesis to mass production and the filing of numerous patents.
“None of the three components — core, shell or ligand can be overlooked,” added Lee. “Samsung’s technology for indium phosphide synthesis is outstanding.”
“Developing a technology in the lab is a challenge in itself, but commercialization requires an entirely different level of effort to ensure product stability and consistent color quality,” said Hyeon. “I was truly impressed that Samsung succeeded in commercializing a no-cadmium quantum dot display product.”
Setting the Quantum Dot Standard
“Research trends in the academic community shifted noticeably before and after the release of Samsung’s quantum dot TVs.”
— Doh Chang Lee, Korea Advanced Institute of Science and Technology
The optical properties of quantum dots are being applied to a wide range of fields, including solar cells, medicine and quantum computing. However, the quantum dot display remains the most actively researched and widely commercialized application to date — with Samsung emerging as a pioneer.
Building on years of foundational research and the introduction of its SUHD TVs, Samsung launched its QLED TVs in 2017 and set a new standard for premium displays. In 2022, the company pushed innovation further with the debut of QD-OLED TVs — the world’s first display to combine quantum dots with an OLED structure.

▲ A comparison of LCD, QLED and QD-OLED structures
QD-OLED is a next-generation display technology that integrates quantum dots into the self-emissive structure of OLED. This approach enables faster response times, deeper blacks and higher contrast ratios. Samsung’s QD-OLED was awarded Display of the Year in 2023 by the Society for Information Display (SID), the world’s largest organization dedicated to display technologies.
“Samsung has not only led the market with its indium phosphide-based quantum dot TVs but also remains the only company to have successfully integrated and commercialized quantum dots in OLEDs,” said Sohn. “By leveraging our leadership in quantum dot technology, we will continue to lead the future of display innovation.”

▲ Doh Chang Lee
“Research trends in the academic community shifted noticeably before and after the release of Samsung’s quantum dot TVs,” said Doh Chang Lee, a professor in the Department of Chemical and Biomolecular Engineering at the Korea Advanced Institute of Science and Technology (KAIST). “Since its launch, discussions have increasingly focused on practical applications rather than the materials themselves, reflecting the potential for real-world implementation through display technologies.”
“There have been many attempts to apply quantum dots in various fields including photocatalysis,” he added. “But these efforts remain in the early stages compared to their use in displays.”
Hyeon also noted that the successful commercialization of Samsung’s quantum dot TVs helped pave the way for Bawendi, Brus and Yekimov to receive the 2023 Nobel Prize in Chemistry.
“One of the most important criteria for the Nobel Prize is the extent to which a technology has contributed to humanity through commercialization,” he said. “Samsung’s QLED represents one of the most significant achievements in nanotechnology. Without its commercialization, it would have been difficult for quantum dots to earn Nobel recognition.”
Samsung’s Vision for Tomorrow’s Displays
Since the launch of its QLED TVs, Samsung has accelerated the growth of quantum dot technology in both industry and academia. When asked about the future of quantum dot displays, the experts shared their insights on what lies ahead.
“As a next-generation technology, we are currently exploring self-emissive quantum dots,” said Sohn. “Until now, quantum dots have relied on external light source to express red and green. Going forward, we aim to develop quantum dots that emit light independently through electroluminescence — producing all three primary colors by injecting electrical energy. We are also working on the development of blue quantum dots.”
“As electroluminescent materials make it possible to reduce the size of device components, we’ll be able to achieve the high resolution, efficiency and brightness required for virtual and augmented reality applications,” said Lee, predicting a major transformation in the future of displays.
“A good display is one the viewer doesn’t even recognize as a display,” said Sohn. “The ultimate goal is to deliver an experience that feels indistinguishable from reality. As a leader in quantum dot display innovation, we will proudly continue to move forward.”
With its continued leadership and bold technological vision, Samsung is shaping the future of displays and rewriting what’s possible with quantum dots.
Five Frequently Asked Questions about QLED TVs
Q. What is Real QLED?
“Samsung’s Real QLED is an innovative display that uses quantum dot (QD) light-converting film combined with blue light sources and is certified as ‘Real Quantum Dot Display’ by TÜV Rheinland, an international certification organization based in Germany. It displays clear separation between red, green and blue light sources — an important marker of color accuracy.
This distinction, enabled by quantum dots, may not be as pronounced in displays using alternative materials or methods with very little quantum dot presence, which can sometimes cause color mixing or reduced clarity. It demonstrates how Samsung’s use of quantum dots contributes to delivering vivid and precise color expression.”
Q. What is a Quantum dot on TV?
Quantum dots are ultra-fine nanomaterials — tens of thousands of times smaller than a human hair — renowned for their ability to reproduce vivid, precise colors depending on the light wavelength. The way quantum dots are integrated into display panels has become a key indicator for evaluating technological advancement in the premium TV segment.
Q. How do I know my TV is a Real QLED?
“Check for the ‘Real Quantum Dot Display’ certification logo from TÜV Rheinland, an international certification organization based in Germany. The certification verifies that Samsung’s QLED TVs meet global standards for quantum dot display structure, confirming that Samsung QLED TVs comply with the International Electrotechnical Commission (IEC) Draft Technical Report 62595-1-6, which describes the application of quantum dot (QD) light converting unit combined with blue light sources for standard QLED displays.
As part of the certification process, TÜV Rheinland analyzed the light spectrum produced by Samsung QLED TVs and confirmed that it displayed clear separation between red, green and blue — an important marker of color accuracy. With this certification, Samsung’s QLED TVs are officially validated as true quantum dot displays, delivering true quantum dot performance built to international standards.”
Q. Why is Samsung’s QLED safer?
Samsung’s quantum dot technology has also been recognized by global testing organization Société Générale de Surveillance (SGS) for its excellence in cadmium-free design — an environmentally conscious approach that eliminates the use of cadmium, a toxic heavy metal known to pose risks to human health and the environment.
Q. Some brands claim that their TVs are QLEDs despite not having any certification. How can they claim this and what should I look out for?
Standard QLED displays are defined by the application of quantum dot (QD) light converting unit combined with blue light sources. Some brands claim that a microscopic dose of quantum dot material on the diffuser plate makes their TVs Real Quantum dot displays. But an independent third-party institution (a university laboratory) tested a subset of the models in question by replacing their diffuser plate with ordinary diffuser plate (i.e., without quantum dots) to examine whether such microscopic dose of quantum dot has any actual effect to amplify, convert or influence the color of light. The test results confirmed that such trace amount of quantum dots made no contribution whatsoever, showing no difference in the color compared to the ordinary diffuser plates. Samsung QLED TVs and Neo QLED TVs have received the “Real Quantum Dot Display” certification from TUV.
1 An ionic bond is a chemical bond formed when electrons are transferred between atoms, creating ions that are held together by electrical attraction.
2 A covalent bond is a chemical bond in which two atoms share electrons.



